

Osteosarcoma is a rare and aggressive bone cancer that primarily affects children and young adults. Lithea’s lead candidate delivers chemotherapy directly into the tumour to improve local control and reduce systemic side effects.
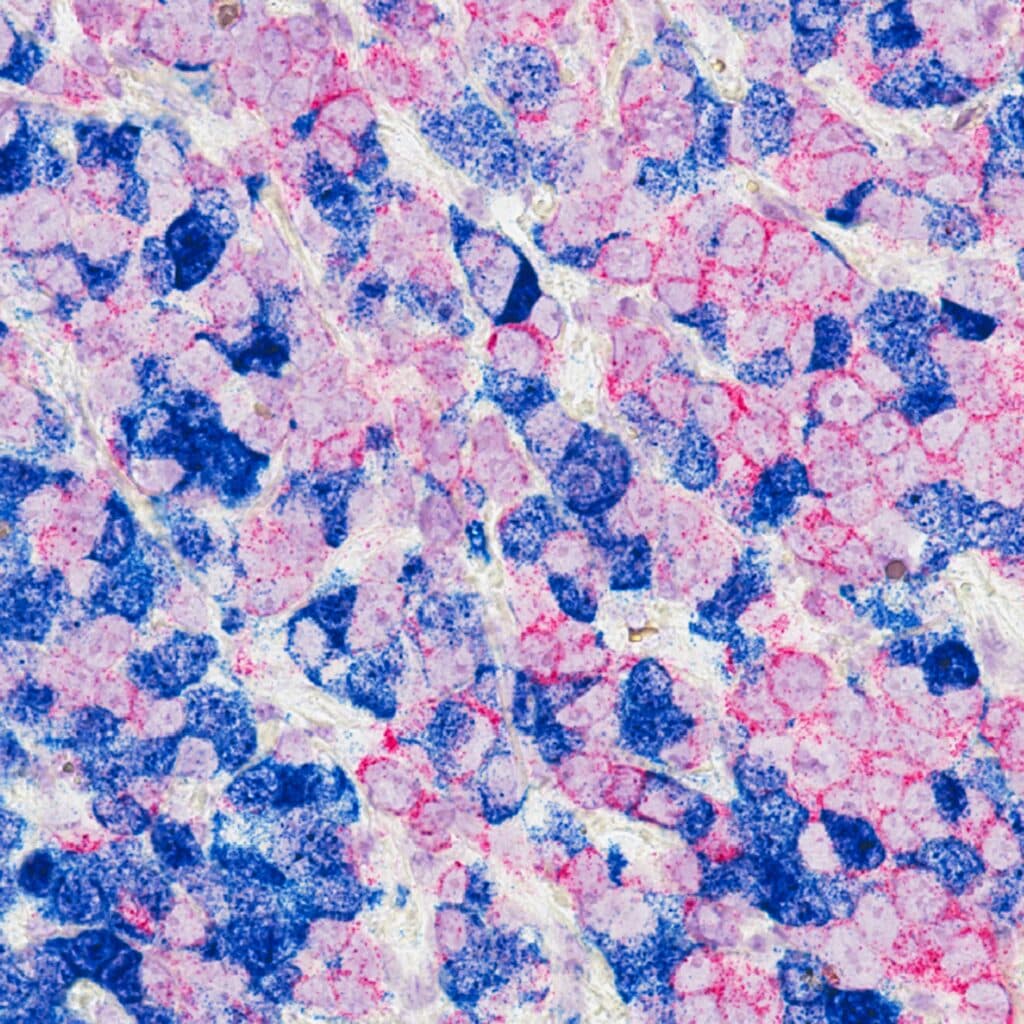
Breast cancer often recurs at the original tumour site despite systemic treatment. Lithea’s platform enables local delivery of chemotherapy during or after surgery to target residual cancer cells and lower the risk of relapse.
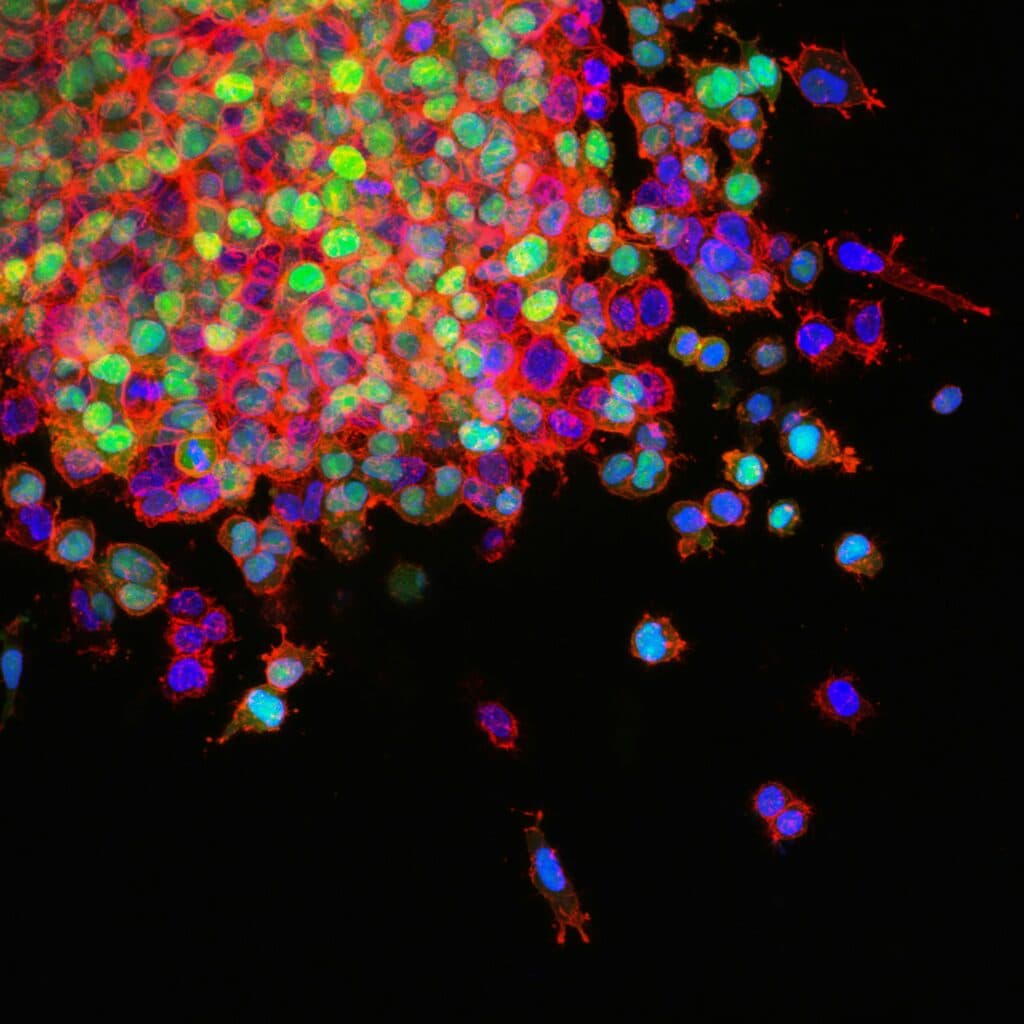
Lung cancer frequently spreads to the bones, where treatment options are limited. Lithea’s implants are designed to deliver drugs directly to bone metastases, increasing local efficacy and reducing systemic toxicity.
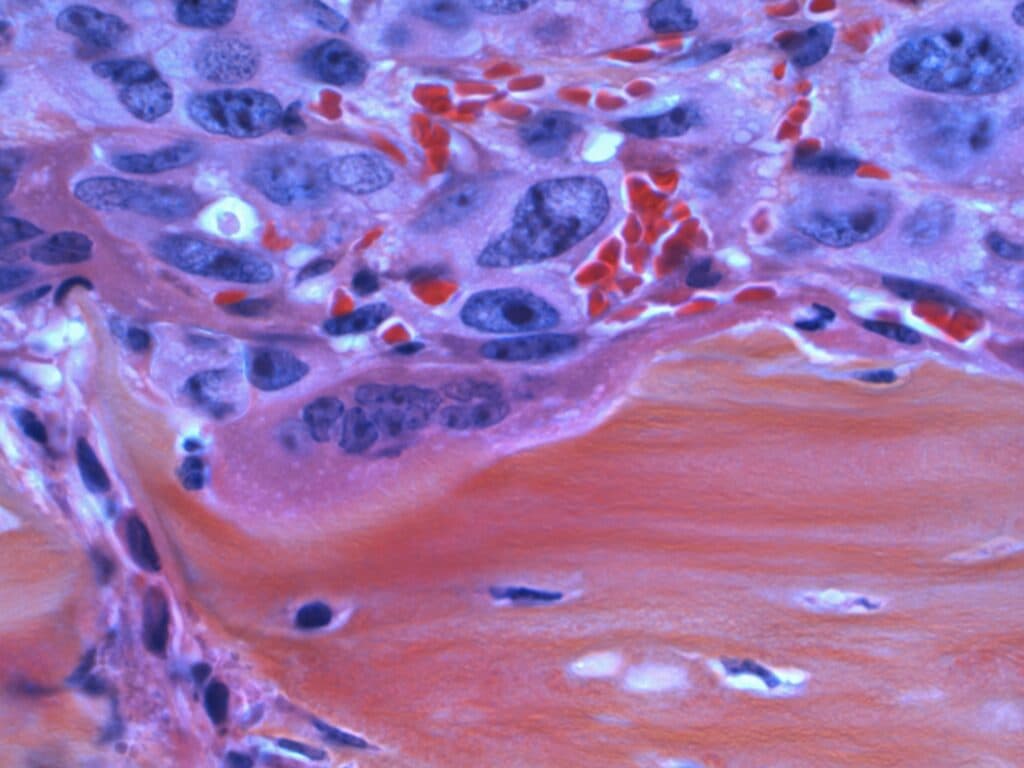
Bladder cancer with bone involvement is difficult to treat with conventional chemotherapy. Lithea’s approach targets these sites with sustained local therapy, aiming to control progression and ease treatment burden.
Osteosarcoma treatment comprises pre-surgical chemotherapy followed by radical surgery and further chemotherapy cycles, but the prognosis has been far from satisfactory. No new drugs or treatment modalities have been developed for clinical use in the last four decades. We describe a nano-hydroxyapatite (HA)-based local drug delivery platform for the delivery of doxorubicin (DOX), a cornerstone drug in osteosarcoma treatment.
The efficacy of the developed drug delivery system was evaluated in an orthotopic human osteosarcoma xenograft in the proximal tibia of mice. After tumor development, the tumor was surgically resected and the void filled with the following: (1) No treatment (G1); (2) nHA only (G2); (3) DOX-loaded nHA (G3). In-vivo tumor response was assessed by evaluating the tumor-induced osteolysis at 2 weeks using micro-CT followed by in-vivo PET-CT at 3 weeks and ex-vivo micro-CT and histology. Micro-CT imaging revealed complete destruction of the tibial metaphysis in groups G1 and G2, while the metaphysis was protected from osteolysis in G3. PET-CT imaging using 18F-FDG revealed high metabolic activity in the tumors in G1 and G2, which was significantly reduced in G3.
Using histology, we were able to verify that local DOX delivery reduced the bone destruction and the tumor burden compared with G1 and G2. No off-target toxicity in the vital organs could be observed in any of the treatment groups histologically. This study describes a novel local drug adjuvant delivery approach that could potentially improve the prognosis for patients responding poorly to the current osteosarcoma treatment.
Full article: https://www.mdpi.com/2079-4983/15/8/232